Heat and Thermodynamics 5 Question 2
2. A sample of an ideal gas is taken through the cyclic process
(2019 Main, 12 April I)
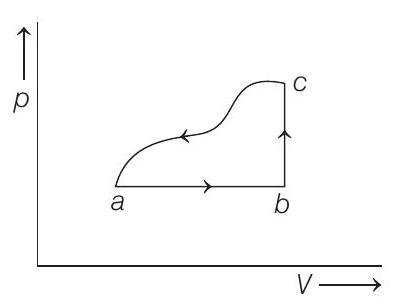
(a)
(b)
(c)
(d)
Show Answer
Solution:
- Key Idea In
and total change in internal energy in complete cycle is always zero. Using this equation in different part of the curve, we can solve the
given problem.
In Process
In Process
Also,
In Process
Given,
Now, for complete cycle,
From Eqs. (iii), (iv) and (v), we get
From Eq. (ii), we get
From Eqs. (i) and (vii), we get
Work done by the gas along the path






