Heat and Thermodynamics 5 Question 18
19. One mole of a monatomic ideal gas is taken along two cyclic processes $E \rightarrow F \rightarrow G \rightarrow E$ and $E \rightarrow F \rightarrow H \rightarrow E$ as shown in the $p-V$ diagram.
(2013 Adv.)
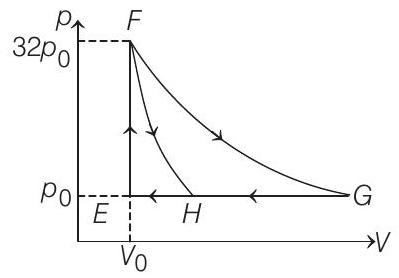
The processes involved are purely isochoric, isobaric, isothermal or adiabatic.
Match the paths in List I with the magnitudes of the work done in List II and select the correct answer using the codes given below the lists.
| List I | List II | ||
|---|---|---|---|
| P. | $G \rightarrow E$ | 1. | $160 p _0 V _0 \ln 2$ |
| Q. | $G \rightarrow H$ | 2. | $36 p _0 V _0$ |
| R. | $F \rightarrow H$ | 3. | $24 p _0 V _0$ |
| S. | $F \rightarrow G$ | 4. | $31 p _0 V _0$ |
Codes
| P | $Q$ | $R$ | $S$ | $P$ | $Q$ | $R$ | $S$ | |
|---|---|---|---|---|---|---|---|---|
| (a) | 4 | 3 | 2 | 1 | (b) 4 | 3 | 1 | 2 |
| (c) | 3 | 1 | 2 | 4 | (d) 1 | 3 | 2 | 4 |
Show Answer
Solution:

In $F \rightarrow G$ work done in isothermal process is
$$ \begin{aligned} n R T \ln \left(\frac{V _f}{V _i}\right) & =32 p _0 V _0 \ln \left(\frac{32 V _0}{V _0}\right) \\ & =32 p _0 V _0 \ln 2^{5}=160 p _0 V _0 \ln 2 \end{aligned} $$
In $G \rightarrow E, \Delta W=p _0 \Delta V=p _0\left(31 V _0\right)=31 p _0 V _0$
In $G \rightarrow H$ work done is less than $31 p _0 V _0$ i.e. $24 p _0 V _0$
$\ln F \rightarrow H$ work done is $36 p _0 V _0$






