Heat and Thermodynamics 1 Question 15
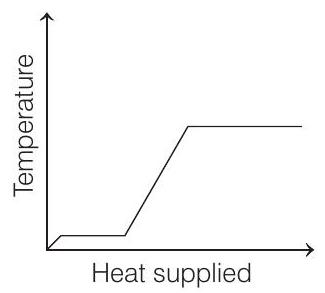
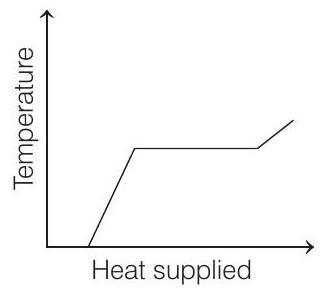
15. A block of ice at
(c)
(b)

(d)

Show Answer
Solution:
- The temperature of ice will first increase from

Then, ice starts melting. Temperature during melting will remain constant
Heat supplied in this process will be
Now, the temperature of water will increase from
where,
Finally, water at






