Thermodynamics and Thermochemistry - Result Question 68
####68. Two moles of a perfect gas undergo the following processes :
(a) a reversible isobaric expansion from $(1.0 atm, 20.0 L)$ to $(1.0 atm, 40.0 L)$ (b) a reversible isochoric change of state from $(1.0 atm$, $40.0 L)$ to $(0.5 atm, 40.0 L)$
(c) a reversible isothermal compression from $(0.5 atm$, $40.0 L)$ to ( $1.0 atm, 20.0 L)$
(i) Sketch with labels each of the processes on the same $p$ - $V$ diagram.
(ii) Calculate the total work $(W)$ and the total heat change $(Q)$ involved in the above processes.
(iii) What will be the values of $\Delta U, \Delta H$ and $\Delta S$ for the overall process?
(2002, 5M)
Show Answer
Solution:
- (i)
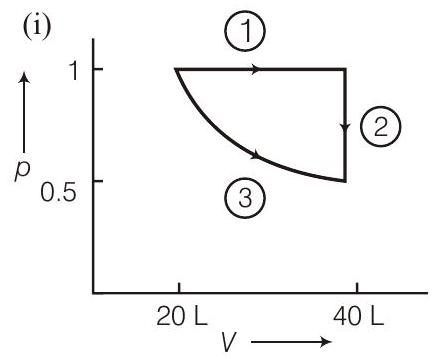
$$ \begin{aligned} -W _1 & =p \Delta V=20 L \text { atm } \ W _2 & =0 \quad \because \quad \Delta V=0 \ W _3 & =n R T \ln \frac{40}{20}=20 \ln 2 \end{aligned} $$
Total work done $=W _1+W _2+W _3$
$$ \begin{aligned} & =-20 L atm+0+20 \ln 2 \ & =-6.14 atm \end{aligned} $$
From first law : $q=\Delta E+(-W)=-W$
$$ \Rightarrow \quad q=6.14 L atm=622.53 J $$
(iii) All the states function, $\Delta U, \Delta H$ and $\Delta S$ are zero for cyclic process.






