Solid State - Result Question 3
####3. Consider the bcc unit cells of the solids 1 and 2 with the position of atoms as shown below. The radius of atom
(2019 Main, 8 April II)
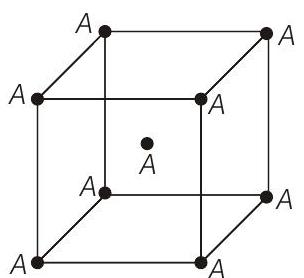
Solid 1
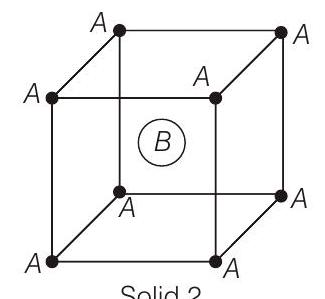
Solid 2
(a)
(b)
(c)
(d)
Show Answer
Answer:
Correct Answer: 3. (b)
Solution:
Key Idea Packing efficiency
Given,
For bcc lattice
[As the atoms






