Solid State - Result Question 15
####15.
(a)
(b)
(c)
(d)
(2014 Main)
Show Answer
Answer:
Correct Answer: 15. (c)
Solution:
- In
Calculation of
In
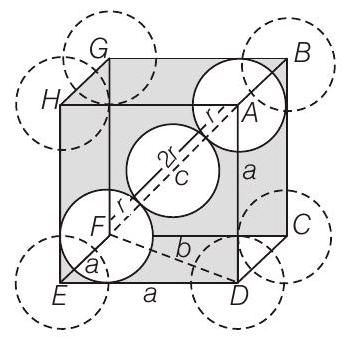
Body centred cubic unit cell
In
As






