####40. For a first order reaction at constant volume and , the total pressure at the beginning and at time are and , respectively. Initially, only is present with concentration , and is the time required for the partial pressure of to reach of its initial value. The correct option(s) is (are) (Assume that all these gases behave as ideal gases)
(2018 Adv.) (a)
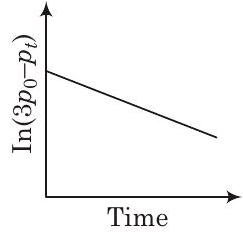
(c)
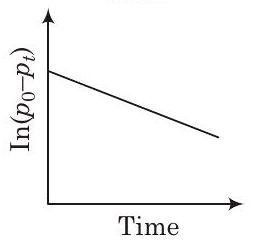
(b)
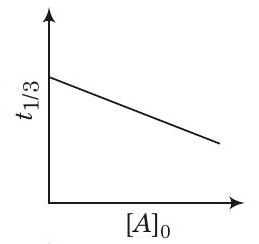
(d)
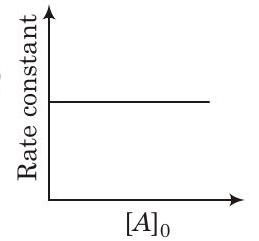
Show Answer
Answer:
Correct Answer: 40.
Solution:
- Given for the reaction (at and constant volume )
We can calculate,
Now for first order reaction,
Putting the value of in the equation,
It indicates graph between ’ ’ will be a straight line with negative slope, so option (a) is correct
It indicates is independent of initial concentration so, option (b) is incorrect.
Likewise, rate constant also does not show its dependence over initial concentration. Thus, graph between rate constant and will be a straight line parallel to -axis










