####28. For a reaction, , the plots of and with time at temperatures and are given below.
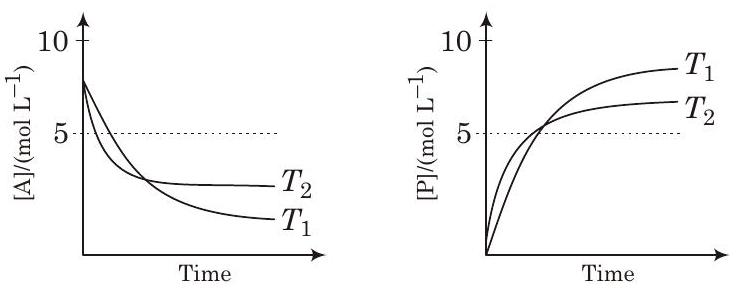
If , the correct statement(s) is are
(Assume and are independent of temperature and ratio of at to at is greater than . Here and are enthalpy, entropy, Gibbs energy and equilibrium constant, respectively.)
(2018 Adv.)
(a)
(b)
(c)
(d)
Show Answer
Solution:
- For the reaction,
Given,
It shows, On increasing the temperature, decreases so reaction is exothermic i.e.,
Besides, graph shows
So
Now from equation (i)
In other words, increase of with increase in temperature is possible only when . Hence, options (a) and (c) are correct.







