Question: Q. 1. The following table shows some measurements of the decay rate of a radio nuclei sample. Find the disintegration constant.
| Time (min) | |
|---|---|
| 36 | 5.08 |
| 100 | 3.29 |
| 164 | 1.52 |
| 218 | 1.00 |
| [SQP 2016] |
Show Answer
Solution:
Ans.
Or
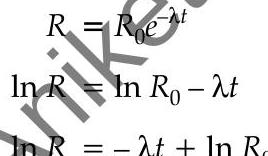
comparing the above equation with line equation






