Question: Q. 11.8, Page 70]
Show Answer
Solution:
Ans. Correct option : (c)
Explanation : According to the problem de-Broglie wavelength of electron at time
Electrostatic force on electron in electric field is
$\vec{F}{e}=-e \vec{E}=-e E{0} \hat{j}$
The acceleration of electron,
It is acting along negative
The initial velocity of electron along
Magnitude of velocity of electron after time
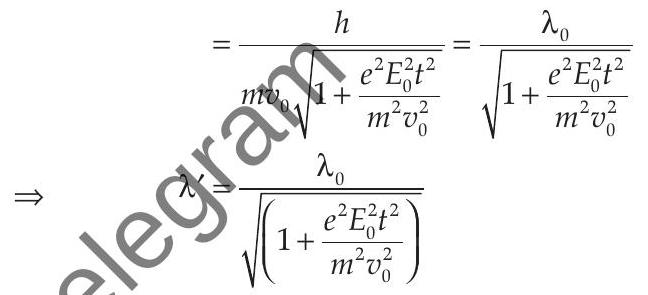
de-Broglie wavelength, at
de-Broglie wavelength at