Question: Q. 1. State Einstein’s photoelectric equation explaining the symbols used.
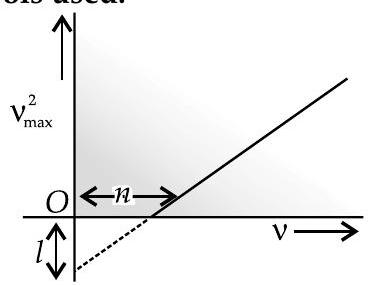
Light of frequency is incident on a photosensitive surface. A graph of the square of the maximum speed of the electrons vs. is obtained as shown in the figure. Using Einstein’s photoelectric equation, obtain expressions for (i) Planck’s constant. (ii) work function of the given photosensitive material in terms of parameters and mass of the electron . [CBSE Comptt. 2018]
Show Answer
Solution:
Ans. Statement of equation with explanation of symbols
Expression for
(i) Planck’s constant
(ii) Work function
1
Einstein’s photoelectric equation is
frequency of incident light
threshold frequency of photo sensitive material
work function
max. kinetic energy of the emitted
photoelectrons
(Also accept if the student writes
work function of photosensitive material
Stopping Potential)
From Einstein’s photoelectric equation, we have
Slope of the given graph
intercept on the -axis
or
and
[CBSE Marking Scheme 2018]







