Question: Q. 3. (i) State Bohr’s quantization condition for defining stationary orbits. How does de-Broglie hypothesis explain the stationary orbits?
(ii) Find the relation between the three wavelengths
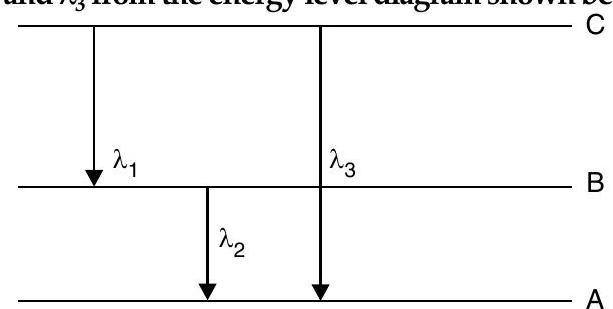
R [Delhi I, II, III 2016]
Show Answer
Solution:
Ans. (i)
According to de-Broglie hypothesis
Linear momentum
And for circular orbit,
Also
(ii)
Adding (i) & (ii)
Using equation (iii) and (iv)
[CBSE Marking Scheme 2016]
Commonly Made Error
- Many students couldn’t understand how to start. They had written the formulae of ‘Balmer, Lyman & Paschen Series.






