Question: Q. 2. Using Bohr’s postulates, derive the expression for the orbital period of the electron moving in the
R [Foreign, I, II, III 2017]
Show Answer
Solution:
Ans.
Also,
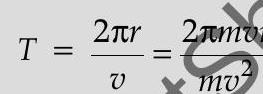
(Also accept if the student calculates
[CBSE Marking Scheme 2017]
Question: Q. 2. Using Bohr’s postulates, derive the expression for the orbital period of the electron moving in the
R [Foreign, I, II, III 2017]
Solution:
Ans.
Also,
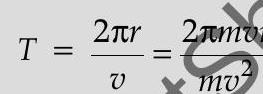
(Also accept if the student calculates
[CBSE Marking Scheme 2017]
© 2024 कॉपीराइट SATHEE
द्वारा संचालित Prutor@IITK
Welcome to SATHEE !
Select from 'Menu' to explore our services, or ask SATHEE to get started. Let's embark on this journey of growth together! 🌐📚🚀🎓
I'm relatively new and can sometimes make mistakes.
If you notice any error, such as an incorrect solution, please use the thumbs down icon to aid my learning.
To begin your journey now, click on "I understand".