The P Block elements
In Class XI, you have learnt that the
Having learnt the chemistry of elements of Groups 13 and 14 of the
7.1 Group 15 Elements
Group 15 includes nitrogen, phosphorus, arsenic, antimony, bismuth and moscovium. As we go down the group, there is a shift from nonmetallic to metallic through metalloidic character. Nitrogen and phosphorus are non-metals, arsenic and antimony metalloids, bismuth and moscovium are typical metals.
7.1.1 Occurrence
Molecular nitrogen comprises
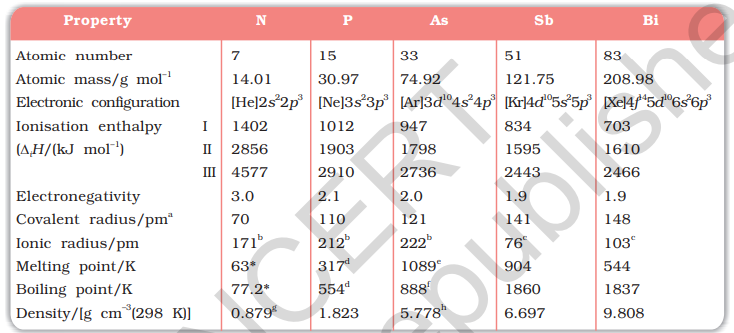
Trends of some of the atomic, physical and chemical properties of the group are discussed below.
7.1.2 Electronic Configuration
The valence shell electronic configuration of these elements is ns2np3. The s orbital in these elements is completely filled and p orbitals are half-filled, making their electronic configuration extra stable.
7.1.3 Atomic and Ionic Radii
Covalent and ionic (in a particular state) radii increase in size down the group. There is a considerable increase in covalent radius from N to P. However, from As to Bi only a small increase in covalent radius is observed. This is due to the presence of completely filled d and/or f orbitals in heavier members.
7.1.4 Ionisation Enthalpy
Ionisation enthalpy decreases down the group due to gradual increase in atomic size. Because of the extra stable half-filled
7.1.5 Electronegativity
The electronegativity value, in general, decreases down the group with increasing atomic size. However, amongst the heavier elements, the difference is not that much pronounced.
7.1.6 Physical Properties
All the elements of this group are polyatomic. Dinitrogen is a diatomic gas while all others are solids. Metallic character increases down the group. Nitrogen and phosphorus are non-metals, arsenic and antimony metalloids and bismuth is a metal. This is due to decrease in ionisation enthalpy and increase in atomic size. The boiling points, in general, increase from top to bottom in the group but the melting point increases upto arsenic and then decreases upto bismuth. Except nitrogen, all the elements show allotropy.
7.1.7 Chemical Properties
Oxidation states and trends in chemical reactivity
The common oxidation states of these elements are
Similarly, in case of phosphorus nearly all intermediate oxidation states disproportionate into +5 and –3 both in alkali and acid. However +3 oxidation state in case of arsenic, antimony and bismuth becomes increasingly stable with respect to disproportionation.
Nitrogen is restricted to a maximum covalency of 4 since only four (one
Anomalous properties of nitrogen
Nitrogen differs from the rest of the members of this group due to its small size, high electronegativity, high ionisation enthalpy and non-availability of
(i) Reactivity towards hydrogen: All the elements of Group 15 form hydrides of the type
Table 7.2: Properties of Hydrides of Group 15 Elements
| Property | PH |
AsH |
SbH |
BiH |
|
|---|---|---|---|---|---|
| Melting point/K | 195.2 | 139.5 | 156.7 | 185 | - |
| Boiling point/K | 238.5 | 185.5 | 210.6 | 254.6 | 290 |
| (E-H) Distance/pm | 101.7 | 141.9 | 151.9 | 170.7 | - |
| HEH angle (') | 107.8 | 93.6 | 91.8 | 91.3 | - |
| -46.1 | 13.4 | 66.4 | 145.1 | 278 | |
| 389 | 322 | 297 | 255 | - |
(ii) Reactivity towards oxygen: All these elements form two types of oxides:
(iii) Reactivity towards halogens: These elements react to form two series of halides:
(iv) Reactivity towards metals: All these elements react with metals to form their binary compounds exhibiting -3 oxidation state, such as,
Exercises
Intext Questions
7.1 Why are pentahalides of P, As, Sb and Bi more covalent than their trihalides?
Show Answer
Answer
In pentahalides, the oxidation state is +5 and in trihalides, the oxidation state is +3 . Since the metal ion with a high charge has more polarizing power, pentahalides are more covalent than trihalides.
Answer
As we move down a group, the atomic size increases and the stability of the hydrides of group 15 elements decreases. Since the stability of hydrides decreases on moving from
7.2 Dinitrogen
Preparation
Dinitrogen is produced commercially by the liquefaction and fractional distillation of air. Liquid dinitrogen (b.p.
In the laboratory, dinitrogen is prepared by treating an aqueous solution of ammonium chloride with sodium nitrite.
Small amounts of
Very pure nitrogen can be obtained by the thermal decomposition of sodium or barium azide.
Properties
Dinitrogen is a colourless, odourless, tasteless and non-toxic gas. Nitrogen atom has two stable isotopes:
Dinitrogen is rather inert at room temperature because of the high bond enthalpy of
It combines with hydrogen at about
Dinitrogen combines with dioxygen only at very high temperature (at about
Uses: The main use of dinitrogen is in the manufacture of ammonia and other industrial chemicals containing nitrogen, (e.g., calcium cyanamide). It also finds use where an inert atmosphere is required (e.g., in iron and steel industry, inert diluent for reactive chemicals). Liquid dinitrogen is used as a refrigerant to preserve biological materials, food items and in cryosurgery.
Example 7.1 Write the reaction of thermal decomposition of sodium azide.
Solution Thermal decomposition of sodium azide gives dinitrogen gas.
Exercises
Intext Question
7.3 Why is
Show Answer
Answer
The two
7.3 Ammonia
Preparation Ammonia is present in small quantities in air and soil where it is formed by the decay of nitrogenous organic matter e.g., urea.
On a small scale ammonia is obtained from ammonium salts which decompose when treated with caustic soda or calcium hydroxide.
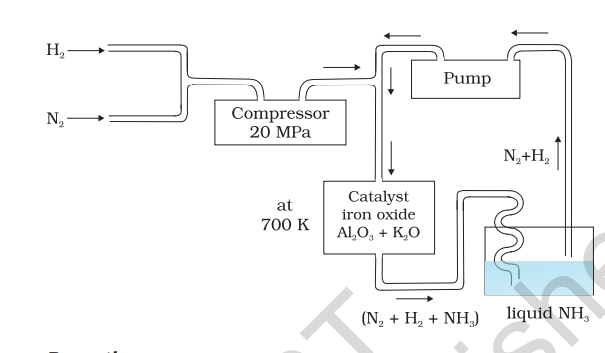
On a large scale, ammonia is manufactured by Haber’s process.
In accordance with Le Chatelier’s principle, high pressure would favour the formation of ammonia. The optimum conditions for the production of ammonia are a pressure of
Properties
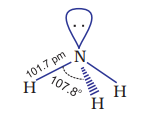
Ammonia is a colourless gas with a pungent odour. Its freezing and boiling points are 198.4 and
Ammonia gas is highly soluble in water. Its aqueous solution is weakly basic due to the formation of
It forms ammonium salts with acids, e.g.,
The presence of a lone pair of electrons on the nitrogen atom of the ammonia molecule makes it a Lewis base. It donates the electron pair and forms linkage with metal ions and the formation of such complex compounds finds applications in detection of metal ions such as
Uses: Ammonia is used to produce various nitrogenous fertilisers (ammonium nitrate, urea, ammonium phosphate and ammonium sulphate) and in the manufacture of some inorganic nitrogen compounds, the most important one being nitric acid. Liquid ammonia is also used as a refrigerant.
Example 7.2 Why does
Solution Nitrogen atom in
Exercises
Intext Questions
7.4 Mention the conditions required to maximise the yield of ammonia.
Show Answer
Answer
Ammonia is prepared using the Haber’s process. The yield of ammonia can be maximized under the following conditions:
(i) High pressure
(ii) A temperature of
(iii) Use of a catalyst such as iron oxide mixed with small amounts of
Show Answer
Answer
Blue
7.4 Oxides of Nitrogen
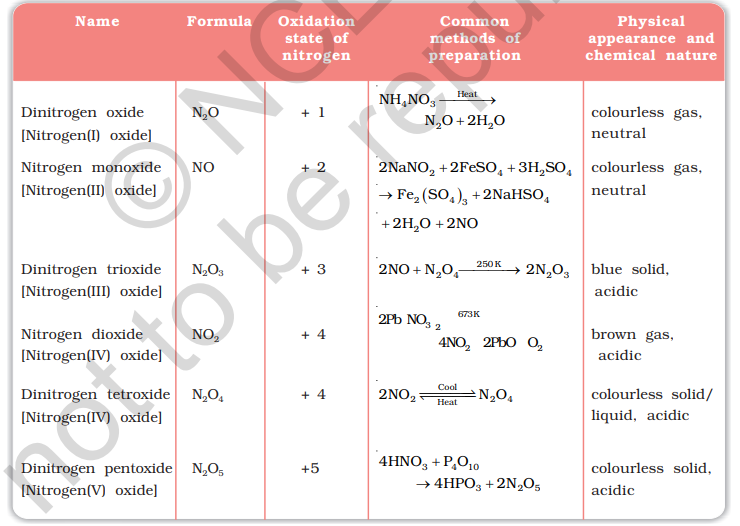
Nitrogen forms a number of oxides in different oxidation states. The names, formulas, preparation and physical appearance of these oxides are given in Table 7.3.
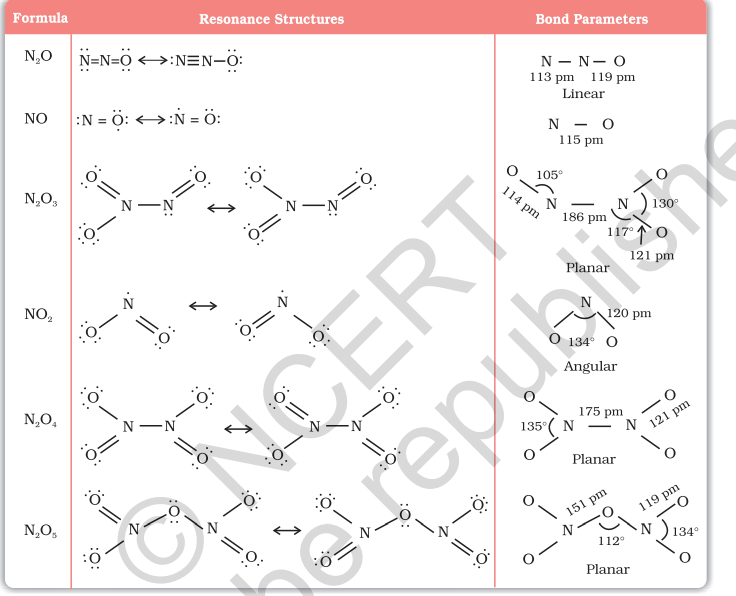
Example 7.3 Why does
Solution
Exercises
Intext Question
7.6 What is the covalence of nitrogen in
Show Answer
Answer

From the structure of
7.5 Nitric Acid
Nitrogen forms oxoacids such as
Preparation In the laboratory, nitric acid is prepared by heating
On a large scale it is prepared mainly by Ostwald’s process.
This method is based upon catalytic oxidation of
Nitric oxide thus formed combines with oxygen giving
Nitrogen dioxide so formed, dissolves in water to give
NO thus formed is recycled and the aqueous
Properties
It is a colourless liquid (f.p.
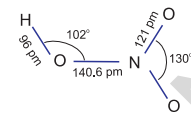
In the gaseous state,
In aqueous solution, nitric acid behaves as a strong acid giving hydronium and nitrate ions.
Concentrated nitric acid is a strong oxidising agent and attacks most metals except noble metals such as gold and platinum. The products of oxidation depend upon the concentration of the acid, temperature and the nature of the material undergoing oxidation.
Zinc reacts with dilute nitric acid to give
Some metals (e.g., Cr, Al) do not dissolve in concentrated nitric acid because of the formation of a passive film of oxide on the surface.
Concentrated nitric acid also oxidises non-metals and their compounds. Iodine is oxidised to iodic acid, carbon to carbon dioxide, sulphur to
Brown Ring Test: The familiar brown ring test for nitrates depends on the ability of
Uses: The major use of nitric acid is in the manufacture of ammonium nitrate for fertilisers and other nitrates for use in explosives and pyrotechnics. It is also used for the preparation of nitroglycerin, trinitrotoluene and other organic nitro compounds. Other major uses are in the pickling of stainless steel, etching of metals and as an oxidiser in rocket fuels.
7.6 Phosphorus — Allotropic Forms
Phosphorus is found in many allotropic forms, the important ones being white, red and black.
White phosphorus is a translucent white waxy solid. It is poisonous, insoluble in water but soluble in carbon disulphide and glows in dark (chemiluminescence). It dissolves in boiling NaOH solution in an inert atmosphere giving PH3.White phosphorus is a translucent white waxy solid. It is poisonous, insoluble in water but soluble in carbon disulphide and glows in dark (chemiluminescence). It dissolves in boiling
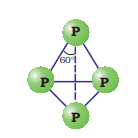
White phosphorus is less stable and therefore, more reactive than the other solid phases under normal conditions because of angular strain in the
It consists of discrete tetrahedral
Red phosphorus is obtained by heating white phosphorus at
It is polymeric, consisting of chains of
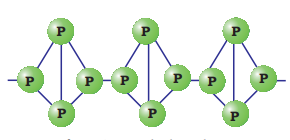
Black phosphorus has two forms
7.7 Phosphine
Preparation Phosphine is prepared by the reaction of calcium phosphide with water or dilute HCl.
In the laboratory, it is prepared by heating white phosphorus with concentrated
When pure, it is non inflammable but becomes inflammable owing to the presence of
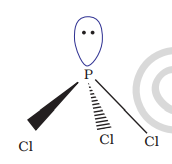
Properties
It is a colourless gas with rotten fish smell and is highly poisonous. It explodes in contact with traces of oxidising agents like
It is slightly soluble in water. The solution of
Uses: The spontaneous combustion of phosphine is technically used in Holme’s signals. Containers containing calcium carbide and calcium phosphide are pierced and thrown in the sea when the gases evolved burn and serve as a signal. It is also used in smoke screens.
Example 7.4
In what way can it be proved that
Solution
Exercises
Intext Questions
7.7 (a) Bond angle in
Show Answer
Answer
In


Show Answer
Answer
White phosphorous dissolves in boiling
7.8 Phosphorus Halides
Phosphorus forms two types of halides,
7.8.1 Phosphorus Trichloride
Preparation It is obtained by passing dry chlorine over heated white phosphorus.
It is also obtained by the action of thionyl chloride with white phosphorus.
Properties It is a colourless oily liquid and hydrolyses in the presence of moisture.
It reacts with organic compounds containing
It has a pyramidal shape as shown, in which phosphorus is
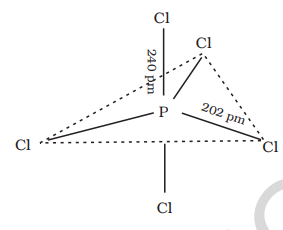
7.8.2 Phosphorus Pentachloride
Preparation Phosphorus pentachloride is prepared by the reaction of white phosphorus with excess of dry chlorine.
Phosphorus pentachloride is prepared by the reaction of white phosphorus with excess of dry chlorine.
It can also be prepared by the action of
Properties
When heated, it sublimes but decomposes on stronger heating.
It reacts with organic compounds containing –OH group converting them to chloro derivatives.
Finely divided metals on heating with
It is used in the synthesis of some organic compounds, e.g.,
In gaseous and liquid phases, it has a trigonal bipyramidal structure as shown. The three equatorial
Example 7.5 Why does
Solution
Example 7.6 Are all the five bonds in
Solution
Exercises
Intext Questions
7.9 What happens when
Show Answer
Answer
All the bonds that are present in
Show Answer
Answer
Therefore, the net reaction can be written as
7.9 Oxoacids of Phosphorus
Phosphorus forms a number of oxoacids. The important oxoacids of phosphorus with their formulas, methods of preparation and the presence of some characteristic bonds in their structures are given in Table 7.5.
Table 7.5: Oxoacids of Phosphorus
| Name | Formula | Oxidation state of phosphorus | Characteristic bonds and their number | Preparation |
|---|---|---|---|---|
| Hypophosphorous (Phosphinic) | +1 | One P - OH Two P - H One P = O | white |
|
| Orthophosphorous (Phosphonic) | +3 | Two P - OH One P - H One P |
||
| Pyrophosphorous | +3 | Two P - OH Two P - H Two P = O | ||
| Hypophosphoric | +4 | Four P |
red |
|
| Orthophosphoric | +5 | Three P |
||
| Pyrophosphoric | 1 | Four |
heat phosphoric acid | |
| Metaphosphoric* | Three |
phosphorus acid |
The compositions of the oxoacids are interrelated in terms of loss or gain of
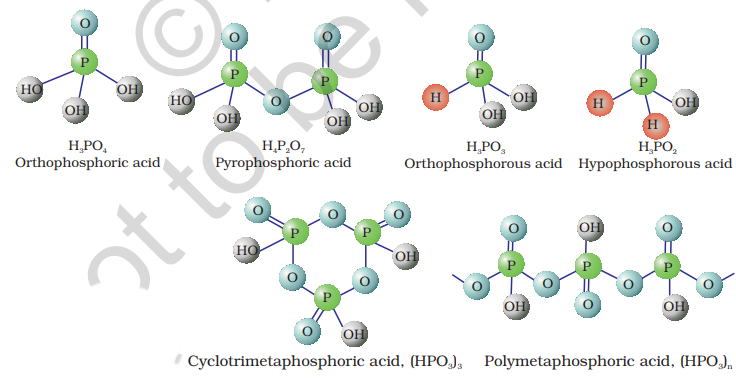
In oxoacids phosphorus is tetrahedrally surrounded by other atoms. All these acids contain at least one
The acids which contain
These
Exercises
Intext Questions
7.11 What is the basicity of
Show Answer
Answer

Since there are three OH groups present in
Show Answer
Answer
7.10 Group 16 Elements
Oxygen, sulphur, selenium, tellurium, polonium and livermorium constitute Group 16 of the periodic table. This is sometimes known as group of chalcogens. The name is derived from the Greek word for brass and points to the association of sulphur and its congeners with copper. Most copper minerals contain either oxygen or sulphur and frequently the other members of the group.
Example 7.7 How do you account for the reducing behaviour of
Solution In
7.10.1 Occurrence
Oxygen is the most abundant of all the elements on earth. Oxygen forms about
However, the abundance of sulphur in the earth’s crust is only 0.03-0.1%. Combined sulphur exists primarily as sulphates such as gypsum
Selenium and tellurium are also found as metal selenides and tellurides in sulphide ores. Polonium occurs in nature as a decay product of thorium and uranium minerals. Livermorium is a synthetic radioactive element. Its symbol is Lv, atomic number 116, atomic mass 292 and electronic configuration [Rn] 5f 146d107s27p4. It has been produced only in a very small amount and has very short half-life (only a small fraction of one second). This limits the study of properlies of Lv.
Here, except for livermorium, important atomic and physical properties of other elements of Group16 along with their electronic configurations are given in Table 7.6. Some of the atomic, physical and chemical properties and their trends are discussed below.
7.10.2 Electronic Configuration
The elements of Group16 have six electrons in the outermost shell and have ns2np4 general electronic configuration.
7.10.3 Atomic and Ionic Radii
Due to increase in the number of shells, atomic and ionic radii increase from top to bottom in the group. The size of oxygen atom is, however, exceptionally small.
7.10.4 Ionisation Enthalpy
Ionisation enthalpy decreases down the group. It is due to increase in size. However, the elements of this group have lower ionisation enthalpy values compared to those of Group15 in the corresponding periods. This is due to the fact that Group 15 elements have extra stable halffilled p orbitals electronic configurations.
7.10.5 Electron Gain Enthalpy
Because of the compact nature of oxygen atom, it has less negative electron gain enthalpy than sulphur. However, from sulphur onwards the value again becomes less negative upto polonium.
7.10.6 Electronegativity
Next to fluorine, oxygen has the highest electronegativity value amongst the elements. Within the group, electronegativity decreases with an increase in atomic number. This implies that the metallic character increases from oxygen to polonium.
Example 7.8
Due to extra stable half-filled
Solution
electrons compared to Group 16 elements.
7.10.7 Physical Properties
Some of the physical properties of Group 16 elements are given in Table 7.6. Oxygen and sulphur are non-metals, selenium and tellurium metalloids, whereas polonium is a metal. Polonium is radioactive and is short lived (Half-life 13.8 days). All these elements exhibit allotropy. The melting and boiling points increase with an increase in atomic number down the group. The large difference between the melting and boiling points of oxygen and sulphur may be explained on the basis of their atomicity; oxygen exists as diatomic molecule (O2) whereas sulphur exists as polyatomic molecule (S8).
7.10.8 Chemical Properties
Oxidation states and trends in chemical reactivity
The elements of Group 16 exhibit a number of oxidation states (Table 7.6). The stability of -2 oxidation state decreases down the group. Polonium hardly shows –2 oxidation state. Since electronegativity of oxygen is very high, it shows only negative oxidation state as –2 except
Table 7.6: Some Physical Properties of Group 16 Elements
| Property | 0 | Se | Te | Po | |
|---|---|---|---|---|---|
| Atomic number | 8 | 16 | 34 | 52 | 84 |
| Atomic mass |
16.00 | 32.06 | 78.96 | 127.60 | 210.00 |
| Electronic configuration | |||||
| Covalent radius |
66 | 104 | 117 | 137 | 146 |
| Ionic radius, |
140 | 184 | 198 | 221 | |
| Electron gain enthalpy, |
-141 | -200 | -195 | -190 | -174 |
| Ionisation enthalpy |
1314 | 1000 | 941 | 869 | 813 |
| Electronegativity | 3.50 | 2.58 | 2.55 | 2.01 | 1.76 |
| Density /g cm |
6.25 | ||||
| Melting point/K | 55 | 490 | 725 | 520 | |
| Boiling point/K | 90 | 718 | 958 | 1260 | 1235 |
| Oxidation states* | 2,4 |
- Oxygen shows oxidation states of +2 and +1 in oxygen fluorides
in the case of
Anomalous behaviour of oxygen
The anomalous behaviour of oxygen, like other members of
The absence of
(i) Reactivity with hydrogen: All the elements of Group 16 form hydrides of the type
Table 7.7: Properties of Hydrides of Group 16 Elements
| Property | ||||
|---|---|---|---|---|
| 273 | 188 | 208 | 222 | |
| b.p/K | 373 | 213 | 232 | 269 |
| 96 | 134 | 146 | 169 | |
| 104 | 92 | 91 | 90 | |
| -286 | -20 | 73 | 100 | |
| 463 | 347 | 276 | 238 | |
| Dissociation constant |
(ii) Reactivity with oxygen: All these elements form oxides of the
(iii) Reactivity towards the halogens: Elements of Group 16 form a large number of halides of the type,
Amongst tetrafluorides,
All elements except oxygen form dichlorides and dibromides. These dihalides are formed by
Example 7.9
Solution
Due to the decrease in bond
Exercises
Intext Questions
7.13 List the important sources of sulphur.
Show Answer
Answer
Sulphur mainly exists in combined form in the earth’s crust primarily as sulphates [gypsum (
Show Answer
Answer
The thermal stability of hydrides decreases on moving down the group. This is due to a decrease in the bond dissociation enthalpy (
Therefore,
Show Answer
Answer
Hence,
7.11 Dioxygen
Preparation Dioxygen can be obtained in the laboratory by the following ways:
(i) By heating oxygen containing salts such as chlorates, nitrates and permanganates.
(ii) By the thermal decomposition of the oxides of metals low in the electrochemical series and higher oxides of some metals.
(iii) Hydrogen peroxide is readily decomposed into water and dioxygen by catalysts such as finely divided metals and manganese dioxide.
On large scale it can be prepared from water or air. Electrolysis of water leads to the release of hydrogen at the cathode and oxygen at the anode.
Industrially, dioxygen is obtained from air by first removing carbon dioxide and water vapour and then, the remaining gases are liquefied and fractionally distilled to give dinitrogen and dioxygen.
Properties
Dioxygen is a colourless and odourless gas. Its solubility in water is to the extent of
Dioxygen directly reacts with nearly all metals and non-metals except some metals ( e.g., Au, Pt) and some noble gases. Its combination with other elements is often strongly exothermic which helps in sustaining the reaction. However, to initiate the reaction, some external heating is required as bond dissociation enthalpy of oxgyen-oxygen double bond is high (
Some of the reactions of dioxygen with metals, non-metals and other compounds are given below:
Some compounds are catalytically oxidised. For example,
Uses: In addition to its importance in normal respiration and combustion processes, oxygen is used in oxyacetylene welding, in the manufacture of many metals, particularly steel. Oxygen cylinders are widely used in hospitals, high altitude flying and in mountaineering. The combustion of fuels, e.g., hydrazines in liquid oxygen, provides tremendous thrust in rockets.
Exercises
Intext Questions
7.16 Which of the following does not react with oxygen directly?
Show Answer
Answer
Pt is a noble metal and does not react very easily. All other elements,
(i)
(ii)
Show Answer
Answer
(i)
(ii)
7.12 Simple Oxides
A binary compound of oxygen with another element is called oxide. As already stated, oxygen reacts with most of the elements of the periodic table to form oxides. In many cases one element forms two or more oxides. The oxides vary widely in their nature and properties.
Oxides can be simple (e.g.,
As a general rule, only non-metal oxides are acidic but oxides of some metals in high oxidation state also have acidic character (e.g.,
In general, metallic oxides are basic.
Some metallic oxides exhibit a dual behaviour. They show characteristics of both acidic as well as basic oxides. Such oxides are known as amphoteric oxides. They react with acids as well as alkalies. For example,
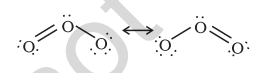
7.13 Ozone
Ozone is an allotropic form of oxygen. It is too reactive to remain for long in the atmosphere at sea level. At a height of about 20 kilometres, it is formed from atmospheric oxygen in the presence of sunlight. This ozone layer protects the earth’s surface from an excessive concentration of ultraviolet (UV) radiations.
Preparation When a slow dry stream of oxygen is passed through a silent electrical discharge, conversion of oxygen to ozone (10%) occurs. The product is known as ozonised oxygen.
Since the formation of ozone from oxygen is an endothermic process, it is necessary to use a silent electrical discharge in its preparation to prevent its decomposition.
If concentrations of ozone greater than 10 per cent are required, a battery of ozonisers can be used, and pure ozone (b.p. 101.1K) can be condensed in a vessel surrounded by liquid oxygen.
Properties Pure ozone is a pale blue gas, dark blue liquid and violet-black solid. Ozone has a characteristic smell and in small concentrations it is harmless. However, if the concentration rises above about 100 parts per million, breathing becomes uncomfortable resulting in headache and nausea.
Ozone is thermodynamically unstable with respect to oxygen since its decomposition into oxygen results in the liberation of heat (
Due to the ease with which it liberates atoms of nascent oxygen
When ozone reacts with an excess of potassium iodide solution buffered with a borate buffer (
Experiments have shown that nitrogen oxides (particularly nitrogen monoxide) combine very rapidly with ozone and there is, thus, the possibility that nitrogen oxides emitted from the exhaust systems of supersonic jet aeroplanes might be slowly depleting the concentration of the ozone layer in the upper atmosphere.
Another threat to this ozone layer is probably posed by the use of freons which are used in aerosol sprays and as refrigerants.
The two oxygen-oxygen bond lengths in the ozone molecule are identical (128 pm) and the molecule is angular as expected with a bond angle of about 117o. It is a resonance hybrid of two main forms:
Uses: It is used as a germicide, disinfectant and for sterilising water. It is also used for bleaching oils, ivory, flour, starch, etc. It acts as an oxidising agent in the manufacture of potassium permanganate.
Exercises
Intext Questions
7.18 Why does
Show Answer
Answer
Ozone is not a very stable compound under normal conditions and decomposes readily on heating to give a molecule of oxygen and nascent oxygen. Nascent oxygen, being a free radical, is very reactive.
Therefore, ozone acts as a powerful oxidising agent.
Show Answer
Answer
Quantitatively, ozone can be estimated with the help of potassium iodide. When ozone is made to react with potassium iodide solution buffered with a borate buffer (
7.14 Sulphur — Allotropic Forms
Sulphur forms numerous allotropes of which the yellow rhombic (
Rhombic sulphur (
This allotrope is yellow in colour, m.p.
Monoclinic sulphur (
Its m.p. is
Both rhombic and monoclinic sulphur have
Several other modifications of sulphur containing 6-20 sulphur atoms per ring have been synthesised in the last two decades. In cyclo-
7.15 Sulphur Dioxide
Preparation Sulphur dioxide is formed together with a little (6-8%) sulphur trioxide when sulphur is burnt in air or oxygen:
In the laboratory it is readily generated by treating a sulphite with dilute sulphuric acid.
Industrially, it is produced as a by-product of the roasting of sulphide ores.
The gas after drying is liquefied under pressure and stored in steel cylinders.
Properties Sulphur dioxide is a colourless gas with pungent smell and is highly soluble in water. It liquefies at room temperature under a pressure of two atmospheres and boils at
Sulphur dioxide, when passed through water, forms a solution of sulphurous acid.
It reacts readily with sodium hydroxide solution, forming sodium sulphite, which then reacts with more sulphur dioxide to form sodium hydrogen sulphite.
In its reaction with water and alkalies, the behaviour of sulphur dioxide is very similar to that of carbon dioxide.
Sulphur dioxide reacts with chlorine in the presence of charcoal (which acts as a catalyst) to give sulphuryl chloride,
When moist, sulphur dioxide behaves as a reducing agent. For example, it converts iron(III) ions to iron(II) ions and decolourises acidified potassium permanganate(VII) solution; the latter reaction is a convenient test for the gas.
The molecule of
Uses: Sulphur dioxide is used (i) in refining petroleum and sugar (ii) in bleaching wool and silk and (iii) as an anti-chlor, disinfectant and preservative. Sulphuric acid, sodium hydrogen sulphite and calcium hydrogen sulphite (industrial chemicals) are manufactured from sulphur dioxide. Liquid SO2 is used as a solvent to dissolve a number of organic and inorganic chemicals.
7.16 Oxoacids of Sulphur
Sulphur forms a number of oxoacids such as
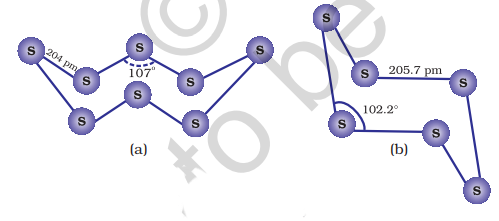
7.17 Sulphuric Acid
Manufacture Sulphuric acid is one of the most important industrial chemicals worldwide. Sulphuric acid is manufactured by the Contact Process which involves three steps:
(i) burning of sulphur or sulphide ores in air to generate
(ii) conversion of
(iii) absorption of
A flow diagram for the manufacture of sulphuric acid is shown in (Fig. 7.7). The
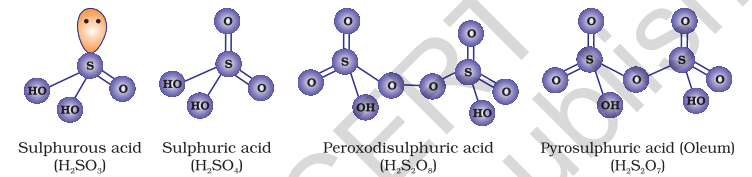
The key step in the manufacture of
The reaction is exothermic, reversible and the forward reaction leads to a decrease in volume. Therefore, low temperature and high pressure are the favourable conditions for maximum yield. But the temperature should not be very low otherwise rate of reaction will become slow.
In practice, the plant is operated at a pressure of 2 bar and a temperature of
The sulphuric acid obtained by Contact process is 96-98% pure.
Properties
Sulphuric acid is a colourless, dense, oily liquid with a specific gravity of 1.84 at
The chemical reactions of sulphuric acid are as a result of the following characteristics: (a) low volatility (b) strong acidic character (c) strong affinity for water and (d) ability to act as an oxidising agent. In aqueous solution, sulphuric acid ionises in two steps.
The larger value of
The acid forms two series of salts: normal sulphates (such as sodium sulphate and copper sulphate) and acid sulphates (e.g., sodium hydrogen sulphate).
Sulphuric acid, because of its low volatility can be used to manufacture more volatile acids from their corresponding salts.
Concentrated sulphuric acid is a strong dehydrating agent. Many wet gases can be dried by passing them through sulphuric acid, provided the gases do not react with the acid. Sulphuric acid removes water from organic compounds; it is evident by its charring action on carbohydrates.
Hot concentrated sulphuric acid is a moderately strong oxidising agent. In this respect, it is intermediate between phosphoric and nitric acids. Both metals and non-metals are oxidised by concentrated sulphuric acid, which is reduced to
Uses: Sulphuric acid is a very important industrial chemical. A nation’s industrial strength can be judged by the quantity of sulphuric acid it produces and consumes. It is needed for the manufacture of hundreds of other compounds and also in many industrial processes. The bulk of sulphuric acid produced is used in the manufacture of fertilisers (e.g., ammonium sulphate, superphosphate). Other uses are in: (a) petroleum refining (b) manufacture of pigments, paints and dyestuff intermediates (c) detergent industry (d) metallurgical applications e.g., cleansing metals before enameling, electroplating and galvanising (e) storage batteries (f) in the manufacture of nitrocellulose products and (g) as a laboratory reagent.
Exercises
Intext Questions
7.23 Mention three areas in which
Show Answer
Answer
Sulphuric acid is an important industrial chemicaland is used for a lot of purposes. Some important uses of sulphuric acid are given below.
(i) It is used in fertilizer industry. It is used to make various fertilizers such as ammonium sulphate and calcium super phosphate.
(ii) It is used in the manufacture of pigments, paints, and detergents.
(iii) It is used in the manufacture of storage batteries.
Show Answer
Answer
Manufacture of sulphuric acid by Contact process involves three steps.
1. Burning of ores to form
2. Conversion of
3. Absorption of
The key step in this process is the second step. In this step, two moles of gaseous reactants combine to give one mole of gaseous product. Also, this reaction is exothermic. Thus, in accordance with Le Chatelier’s principle, to obtain the maximum amount of
Show Answer
Answer
This is because a neutral
7.18 Group 17 Elements
Fluorine, chlorine, bromine, iodine, astatine and tennessine are members of Group 17. These are collectively known as the halogens (Greek halo means salt and genes means born i.e., salt producers). The halogens are highly reactive non-metallic elements. Like Groups 1 and 2, the elements of Group 17 show great similarity amongst themselves. That much similarity is not found in the elements of other groups of the periodic table. Also, there is a regular gradation in their physical and chemical properties. Astatine and tennessine are radioactive elements.
7.18.1 Occurrence
Fluorine and chlorine are fairly abundant while bromine and iodine less so. Fluorine is present mainly as insoluble fluorides (fluorspar
The important atomic and physical properties of Group 17 elements along with their electronic configurations are given in Table 7.8.
Table 7.8: Atomic and Physical Properties of Halogens
| Property | Cl | Br | I | ||
|---|---|---|---|---|---|
| Atomic number | 9 | 17 | 35 | 53 | 85 |
| Atomic mass |
19.00 | 35.45 | 79.90 | 126.90 | 210 |
| Electronic configuration | |||||
| Covalent radius/pm | 64 | 99 | 114 | 133 | - |
| Ionic radius |
133 | 184 | 196 | 220 | - |
| Ionisation enthalpy |
1680 | 1256 | 1142 | 1008 | - |
| Electron gain enthalpy |
-333 | -349 | -325 | -296 | - |
| Electronegativity |
4 | 3.2 | 3.0 | 2.7 | 2.2 |
| 515 | 381 | 347 | 305 | - | |
| - | |||||
| Melting point/K | 54.4 | 172.0 | 265.8 | 386.6 | - |
| Boiling point/K | 84.9 | 239.0 | 332.5 | 458.2 | - |
| Density |
- | ||||
| Distance |
143 | 199 | 228 | 266 | - |
| Bond dissociation enthalpy |
158.8 | 242.6 | 192.8 | 151.1 | - |
| 2.87 | 1.36 | 1.09 | 0.54 | - |
The trends of some of the atomic, physical and chemical properties are discussed below.
Here important atomic and physical properties of Group 17 elements other than tennessine are given along with their electronic configurations [Table 7.8, page 198]. Tennessine is a synthetic radioactive element. Its symbol is Ts, atomic number 117, atomic mass 294 and electronic configuration [Rn] 5f 146d107s27p5. Only very small amount of the element could be prepared. Also its half life is in milliseconds only. That is why its chemistry could not be established.
7.18.2 Electronic Configuration
All these elements have seven electrons in their outermost shell (ns2np5) which is one electron short of the next noble gas.
7.18.3 Atomic and Ionic Radii
The halogens have the smallest atomic radii in their respective periods due to maximum effective nuclear charge. The atomic radius of fluorine like the other elements of second period is extremely small. Atomic and ionic radii increase from fluorine to iodine due to increasing number of quantum shells.
7.18.4 Ionisation Enthalpy
They have little tendency to lose electron. Thus they have very high ionisation enthalpy. Due to increase in atomic size, ionisation enthalpy decreases down the group.
7.18.5 Electron Gain Enthalpy
Halogens have maximum negative electron gain enthalpy in the corresponding periods. This is due to the fact that the atoms of these elements have only one electron less than stable noble gas configurations. Electron gain enthalpy of the elements of the group becomes less negative down the group. However, the negative electron gain enthalpy of fluorine is less than that of chlorine. It is due to small size of fluorine atom. As a result, there are strong interelectronic repulsions in the relatively small 2p orbitals of fluorine and thus, the incoming electron does not experience much attraction.
7.18.6 Electronegativity
They have very high electronegativity. The electronegativity decreases down the group. Fluorine is the most electronegative element in the periodic table.
7.18.7 Physical Properties
Halogens display smooth variations in their physical properties. Fluorine and chlorine are gases, bromine is a liquid and iodine is a solid. Their melting and boiling points steadily increase with atomic number. All halogens are coloured. This is due to absorption of radiations in visible region which results in the excitation of outer electrons to higher energy level. By absorbing different quanta of radiation, they display different colours. For example,
One curious anomaly we notice from Table 7.8 is the smaller enthalpy of dissociation of
Example 7.11
Although electron gain enthalpy of fluorine is less negative as compared to chlorine, fluorine is a stronger oxidising agent than chlorine. Why?
Solution It is due to
(i) low enthalpy of dissociation of F-F bond (Table 7.8).
(ii) high hydration enthalpy of
7.18.8 Chemical Properties
All the halogens exhibit -1 oxidation state. However, chlorine, bromine and iodine exhibit
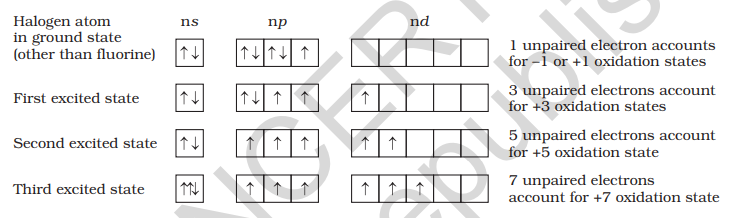
The higher oxidation states of chlorine, bromine and iodine are realised mainly when the halogens are in combination with the small and highly electronegative fluorine and oxygen atoms. e.g., in interhalogens, oxides and oxoacids. The oxidation states of +4 and +6 occur in the oxides and oxoacids of chlorine and bromine. The fluorine atom has no d orbitals in its valence shell and therefore cannot expand its octet. Being the most electronegative, it exhibits only –1 oxidation state.
All the halogens are highly reactive. They react with metals and non-metals to form halides. The reactivity of the halogens decreases down the group.
The ready acceptance of an electron is the reason for the strong oxidising nature of halogens.
The decreasing oxidising ability of the halogens in aqueous solution down the group is evident from their standard electrode potentials (Table 7.8) which are dependent on the parameters indicated below:
The relative oxidising power of halogens can further be illustrated by their reactions with water. Fluorine oxidises water to oxygen whereas chlorine and bromine react with water to form corresponding hydrohalic and hypohalous acids. The reaction of iodine with water is nonspontaneous. In fact, I– can be oxidised by oxygen in acidic medium; just the reverse of the reaction observed with fluorine.
Anomalous behaviour of fluorine
Like other elements of p-block present in second period of the periodic table, fluorine is anomalous in many properties. For example, ionisation enthalpy, electronegativity, and electrode potentials are all higher for fluorine than expected from the trends set by other halogens. Also, ionic and covalent radii, m.p. and b.p., enthalpy of bond dissociation and electron gain enthalpy are quite lower than expected. The anomalous behaviour of fluorine is due to its small size, highest electronegativity, low F-F bond dissociation enthalpy, and non availability of d orbitals in valence shell.
Most of the reactions of fluorine are exothermic (due to the small and strong bond formed by it with other elements). It forms only one oxoacid while other halogens form a number of oxoacids. Hydrogen fluoride is a liquid (b.p. 293 K) due to strong hydrogen bonding. Hydrogen bond is formed in HF due to small size and high electronegativity of fluorine. Other hydrogen halides which have bigger size and less electronegativity are gases.
Most of the reactions of fluorine are exothermic (due to the small and strong bond formed by it with other elements). It forms only one oxoacid while other halogens form a number of oxoacids. Hydrogen fluoride is a liquid (b.p.
(i) Reactivity towards hydrogen: They all react with hydrogen to give hydrogen halides but affinity for hydrogen decreases from fluorine to iodine. Hydrogen halides dissolve in water to form hydrohalic acids. Some of the properties of hydrogen halides are given in Table 7.9. The acidic strength of these acids varies in the order:
Table 7.9: Properties of Hydrogen Halides
| Property | HF | HCl | HBr | HI |
|---|---|---|---|---|
| Melting point/K | 190 | 159 | 185 | 222 |
| Boiling point/K | 293 | 189 | 206 | 238 |
| Bond length |
91.7 | 127.4 | 141.4 | 160.9 |
| 574 | 432 | 363 | 295 | |
| 3.2 | -7.0 | -9.5 | -10.0 |
(ii) Reactivity towards oxygen: Halogens form many oxides with oxygen but most of them are unstable. Fluorine forms two oxides
Chlorine, bromine and iodine form oxides in which the oxidation states of these halogens range from +1 to +7 . A combination of kinetic and thermodynamic factors lead to the generally decreasing order of stability of oxides formed by halogens,
Chlorine oxides,
The bromine oxides,
The iodine oxides,
(iii) Reactivity towards metals: Halogens react with metals to form metal halides. For example, bromine reacts with magnesium to give magnesium bromide.
The ionic character of the halides decreases in the order MF >
(iv) Reactivity of halogens towards other halogens: Halogens combine amongst themselves to form a number of compounds known as interhalogens of the types
Example 7.12
Fluorine exhibits only -1 oxidation state whereas other halogens exhibit
Solution
Fluorine is the most electronegative element and cannot exhibit any positive oxidation state. Other halogens have
Exercises
Intext Questions
7.26 Considering the parameters such as bond dissociation enthalpy, electron gain enthalpy and hydration enthalpy, compare the oxidising power of
Show Answer
Answer
Fluorine is a much stronger oxidizing agent than chlorine. The oxidizing power depends on three factors.
1. Bond dissociation energy
2. Electron gain enthalpy
3. Hydration enthalpy
The electron gain enthalpy of chlorine is more negative than that of fluorine. However, the bond dissociation energy of fluorine is much lesser than that of chlorine. Also, because of its small size, the hydration energy of fluorine is much higher than that of chlorine. Therefore, the latter two factors more than compensate for the less negative electron gain enthalpy of fluorine. Thus, fluorine is a much stronger oxidizing agent than chlorine.
Show Answer
Answer
Anomalous behaviour of fluorine
(i) It forms only one oxoacid as compared to other halogens that form a number of oxoacids.
(ii) Ionisation enthalpy, electronegativity, and electrode potential of fluorine are much higher than expected.
Show Answer
Answer
Sea water contains chlorides, bromides, and iodides of
7.19 Chlorine
Chlorine was discovered in 1774 by Scheele by the action of HCl on MnO2. In 1810 Davy established its elementary nature and suggested the name chlorine on account of its colour (Greek, chloros = greenish yellow).
Preparation
It can be prepared by any one of the following methods:
(i) By heating manganese dioxide with concentrated hydrochloric acid.
However, a mixture of common salt and concentrated
(ii) By the action of
Manufacture of chlorine
(i) Deacon’s process: By oxidation of hydrogen chloride gas by atmospheric oxygen in the presence of
(ii) Electrolytic process: Chlorine is obtained by the electrolysis of brine (concentrated
Properties It is a greenish yellow gas with pungent and suffocating odour. It is about 2-5 times heavier than air. It can be liquefied easily into greenish yellow liquid which boils at
Chlorine reacts with a number of metals and non-metals to form chlorides.
It has great affinity for hydrogen. It reacts with compounds containing hydrogen to form
With excess ammonia, chlorine gives nitrogen and ammonium chloride whereas with excess chlorine, nitrogen trichloride (explosive) is formed.
With cold and dilute alkalies chlorine produces a mixture of chloride and hypochlorite but with hot and concentrated alkalies it gives chloride and chlorate.
(cold and dilute)
(hot and conc.)
With dry slaked lime it gives bleaching powder.
The composition of bleaching powder is
Chlorine reacts with hydrocarbons and gives substitution products with saturated hydrocarbons and addition products with unsaturated hydrocarbons. For example,
CH4 + Cl2 -> CH3Cl + HCl Methane Methyl chloride C2H4 + Cl2 Roomtemp. -> C2H4Cl2 Ethene 1,2-Dichloroethane
Chlorine water on standing loses its yellow colour due to the formation of
(i) It oxidises ferrous to ferric and sulphite to sulphate. Chlorine oxidises sulphur dioxide to sulphur trioxide and iodine to iodate. In the presence of water they form sulphuric acid and iodic acid respectively.
(ii) It is a powerful bleaching agent; bleaching action is due to oxidation.
Coloured substance
Uses: It is used
(i) for bleaching woodpulp (required for the manufacture of paper and rayon), bleaching cotton and textiles,
(ii) in the extraction of gold and platinum (iii) in the manufacture of dyes, drugs and organic compounds such as
Example 7.13
Write the balanced chemical equation for the reaction of
Solution
Yes, chlorine from zero oxidation state is changed to -1 and +5 oxidation states.
Exercises
Intext Questions
7.29 Give the reason for bleaching action of
Show Answer
Answer
When chlorine reacts with water, it produces nascent oxygen. This nascent oxygen then combines with the coloured substances present in the organic matter to oxide them into colourless substances.
Coloured substances
Show Answer
Answer
Two poisonous gases that can be prepared from chlorine gas are
(i) Phosgene
(ii) Mustard gas
7.20 Hydrogen Chloride
Glauber prepared this acid in 1648 by heating common salt with concentrated sulphuric acid. Davy in 1810 showed that it is a compound of hydrogen and chlorine.
Preparation In laboratory, it is prepared by heating sodium chloride with concentrated sulphuric acid.
HCl gas can be dried by passing through concentrated sulphuric acid.
Properties It is a colourless and pungent smelling gas. It is easily liquefied to a colourless liquid (b.p.
Its aqueous solution is called hydrochloric acid. High value of dissociation constant
When three parts of concentrated
Hydrochloric acid decomposes salts of weaker acids, e.g., carbonates, hydrogencarbonates, sulphites, etc.
Uses: It is used (i) in the manufacture of chlorine,
7.21 Oxoacids of Halogens
Due to high electronegativity and small size, fluorine forms only one oxoacid, HOF known as fluoric (I) acid or hypofluorous acid. The other halogens form several oxoacids. Most of them cannot be isolated in pure state. They are stable only in aqueous solutions or in the form of their salts. The oxoacids of halogens are given in Table 7.10 and their structures are given in Fig. 7.8.
Table 7.10: Oxoacids of Halogens
| Halic (I) acid (Hypohalous acid) | HOF (Hypofluorous acid) | HOCl (Hypochlorous acid) | HOBr (Hypobromous acid) | HOI (Hypoiodous acid) |
|---|---|---|---|---|
| Halic (III) acid (Halous acid) | - | HOCIO (chlorous acid) | - | - |
| Halic (V) acid (Halic acid) | - | |||
| Halic (VII) acid (Perhalic acid) | - |
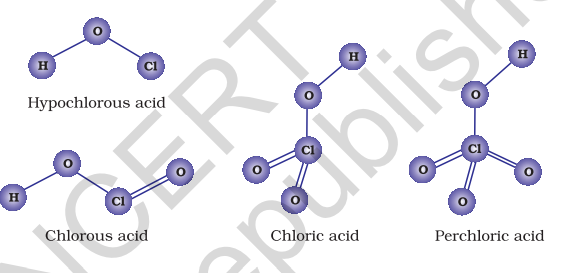
7.22 Interhalogen Compounds
When two different halogens react with each other, interhalogen compounds are formed. They can be assigned general compositions as
Preparation The interhalogen compounds can be prepared by the direct combination or by the action of halogen on lower interhalogen compounds. The product formed depends upon some specific conditions, For example,
Properties Some properties of interhalogen compounds are given in Table 7.11
These are all covalent molecules and are diamagnetic in nature. They are volatile solids or liquids at
Their chemical reactions can be compared with the individual halogens. In general, interhalogen compounds are more reactive than halogens (except fluorine). This is because
Their molecular structures are very interesting which can be explained on the basis of VSEPR theory (Example 7.14). The
Example 7.14 Discuss the molecular shape of
Solution The central atom
Uses: These compounds can be used as non aqueous solvents. Interhalogen compounds are very useful fluorinating agents.
Exercises
Intext Question
7.31 Why is
Show Answer
Answer
7.23 Group 18 Elements
Group 18 consists of elements: helium, neon, argon, krypton, xenon, radon and oganesson. All these are gases and chemically unreactive. They form very few compounds, because of this they are termed as noble gases.
7.23.1 Occurrence
All these gases except radon and oganesson occur in the atmosphere. Their atmospheric abundance in dry air is ~ 1% by volume of which argon is the major constituent. Helium and sometimes neon are found in minerals of radioactive origin e.g., pitchblende, monazite, cleveite. The main commercial source of helium is natural gas. Xenon and radon are the rarest elements of the group. Radon is obtained as a decay product of
Example 7.15 Why are the elements of Group 18 known as noble gases ?
Solution The elements present in Group 18 have their valence shell orbitals completely filled and, therefore, react with a few elements only under certain conditions. Therefore, they are now known as noble gases.
The important atomic and physical properties of the Group 18 elements along with their electronic configurations are given in Table 7.12. The trends in some of the atomic, physical and chemical properties of the group are discussed here.
Table 7.12: Atomic and Physical Properties of Group 18 Elements
| Propery | Ar | |||||
|---|---|---|---|---|---|---|
| Atomic number | 2 | 10 | 18 | 36 | 54 | 86 |
| Atomic mass |
4.00 | 20.18 | 39.95 | 83.80 | 131.30 | 222.00 |
| Electronic configuration | ||||||
| Atomic radius/pm | 120 | 160 | 190 | 200 | 220 | - |
| Ionisation enthalpy |
2372 | 2080 | 1520 | 1351 | 1170 | 1037 |
| Electron gain enthalpy |
48 | 116 | 96 | 96 | 77 | 68 |
| Density (at STP) |
||||||
| Melting point/K | - | 24.6 | 83.8 | 115.9 | 161.3 | 202 |
| Boiling point/K | 4.2 | 27.1 | 87.2 | 119.7 | 165.0 | 211 |
| Atmospheric content (% by volume) | - | 0.934 |
7.23.2 Electronic Configuration
All noble gases have general electronic configuration
7.23.3 Ionisation Enthalpy
Due to stable electronic configuration these gases exhibit very high ionisation enthalpy. However, it decreases down the group with increase in atomic size.
7.23.4 Atomic Radii
Atomic radii increase down the group with increase in atomic number.
7.23.5 Electron Gain Enthalpy
Since noble gases have stable electronic configurations, they have no tendency to accept the electron and therefore, have large positive values of electron gain enthalpy.
Physical Properties
All the noble gases are monoatomic. They are colourless, odourless and tasteless. They are sparingly soluble in water. They have very low melting and boiling points because the only type of interatomic interaction in these elements is weak dispersion forces. Helium has the lowest boiling point (
Chemical Properties In general, noble gases are least reactive. Their inertness to chemical reactivity is attributed to the following reasons:
(i) The noble gases except helium
(ii) They have high ionisation enthalpy and more positive electron gain enthalpy.
The reactivity of noble gases has been investigated occasionally, ever since their discovery, but all attempts to force them to react to form the compounds, were unsuccessful for quite a few years. In March 1962, Neil Bartlett, then at the University of British Columbia, observed the reaction of a noble gas. First, he prepared a red compound which is formulated as
The compounds of krypton are fewer. Only the difluoride
(a) Xenon-fluorine compounds
Xenon forms three binary fluorides,
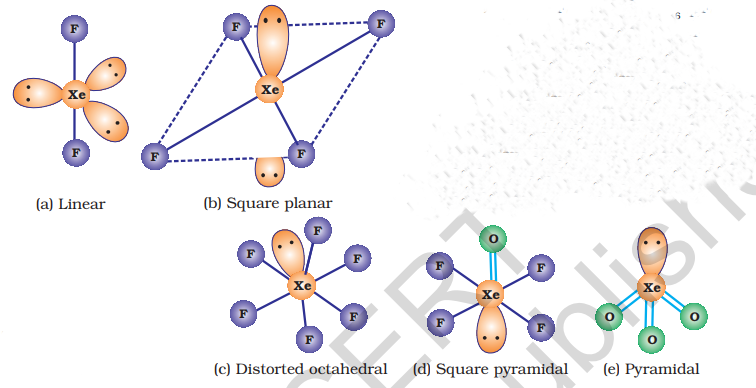
The structures of the three xenon fluorides can be deduced from VSEPR and these are shown in Fig. 7.9.
Xenon fluorides react with fluoride ion acceptors to form cationic species and fluoride ion donors to form fluoroanions.
(b) Xenon-oxygen compounds
Hydrolysis of
Partial hydrolysis of
Uses: Helium is a non-inflammable and light gas. Hence, it is used in filling balloons for meteorological observations. It is also used in gas-cooled nuclear reactors. Liquid helium (b.p.
Neon is used in discharge tubes and fluorescent bulbs for advertisement display purposes. Neon bulbs are used in botanical gardens and in green houses.
Argon is used mainly to provide an inert atmosphere in high temperature metallurgical processes (arc welding of metals or alloys) and for filling electric bulbs. It is also used in the laboratory for handling substances that are air-sensitive. There are no significant uses of Xenon and Krypton. They are used in light bulbs designed for special purposes.
Exercises
Intext Questions
7.32 Why is helium used in diving apparatus?
Show Answer
Answer
Air contains a large amount of nitrogen and the solubility of gases in liquids increases with increase in pressure. When sea divers dive deep into the sea, large amount of nitrogen dissolves in their blood. When they come back to the surface, solubility of nitrogen decreases and it separates from the blood and forms small air bubbles. This leads to a dangerous medical condition called bends. Therefore, air in oxygen cylinders used for diving is diluted with helium gas. This is done as He is sparingly less soluble in blood.
Show Answer
Answer
Balanced equation
Show Answer
Answer
It is difficult to study the chemistry of radon because it is a radioactive substance having a half-life of only 3.82 days. Also, compounds of radon such as
Summary
Groups 13 to 18 of the periodic table consist of -block elements with their valence shell electronic configuration
Group 15 consists of five elements namely, N, P, As, Sb and Bi which have general electronic configuration
Dinitrogen can be prepared in laboratory as well as on industrial scale. It forms oxides in various oxidation states as
Phosphorus exists as
The Group 16 elements have general electronic configuration
Group 17 of the periodic table consists of the following elements F, Cl, Br, I and At.These elements are extremely reactive and as such they are found in the combined state only. The common oxidation state of these elements is -1 . However, highest oxidation state can be +7 . They show regular gradation in physical and chemical properties. They form oxides, hydrogen halides, interhalogen compounds and oxoacids. Chlorine is conveniently obtained by the reaction of
Group 18 of the periodic table consists of noble gases. They have
Due to complete octet of outermost shell, they have less tendency to form compounds. The best characterised compounds are those of xenon with fluorine and oxygen only under certain conditions. These gases have several uses. Argon is used to provide inert atmosphere, helium is used in filling balloons for meteorological observations, neon is used in discharge tubes and fluorescent bulbs.
Exercises
7.1 Discuss the general characteristics of Group 15 elements with reference to their electronic configuration, oxidation state, atomic size, ionisation enthalpy and electronegativity.
Show Answer
Answer
General trends in group 15 elements
(i) Electronic configuration: All the elements in group 15 have 5 valence electrons. Their general electronic configuration is
(ii) Oxidation states: All these elements have 5 valence electrons and require three more electrons to complete their octets. However, gaining electrons is very difficult as the nucleus will have to attract three more electrons. This can take place only with nitrogen as it is the smallest in size and the distance between the nucleus and the valence shell is relatively small. The remaining elements of this group show a formal oxidation state of -3 in their covalent compounds. In addition to the -3 state,
All the elements present in this group show +3 and +5 oxidation states. However, the stability of +5 oxidation state decreases down a group, whereas the stability of +3 oxidation state increases. This happens because of the inert pair effect.
(iii) lonization energy and electronegativity
First ionization decreases on moving down a group. This is because of increasing atomic sizes. As we move down a group, electronegativity decreases, owing to an increase in size.
(iv) Atomic size: On moving down a group, the atomic size increases. This increase in the atomic size is attributed to an increase in the number of shells.
Show Answer
Answer
Nitrogen is chemically less reactive. This is because of the high stability of its molecule,
Show Answer
Answer
General trends in chemical properties of group - 15
(i) Reactivity towards hydrogen:
The elements of group 15 react with hydrogen to form hydrides of type
(ii) Reactivity towards oxygen:
The elements of group 15 form two types of oxides:
(iii) Reactivity towards halogens:
The group 15 elements react with halogens to form two series of salts:
(iv) Reactivity towards metals:
The group 15 elements react with metals to form binary compounds in which metals exhibit -3 oxidation states.
Show Answer
Answer
Nitrogen is highly electronegative as compared to phosphorus. This causes a greater attraction of electrons towards nitrogen in
Show Answer
Answer
An aqueous solution of ammonium chloride is treated with sodium nitrite.
Show Answer
Answer
Ammonia is prepared on a large-scale by the Haber’s process.
Show Answer
Answer
Concentrated nitric acid is a strong oxidizing agent. It is used for oxidizing most metals. The products of oxidation depend on the concentration of the acid, temperature, and also on the material undergoing oxidation.
Show Answer
Answer
(1)

(2)

Show Answer
Answer
Hydride
The above trend in the
Show Answer
Answer
Show Answer
Answer
Nitrogen has a small size due to which the lone pair of electrons is concentrated in a small region. This means that the charge density per unit volume is high. On moving down a group, the size of the central atom increases and the charge gets distributed over a large area decreasing the electron density. Hence, the electron donating capacity of group 15 element hydrides decreases on moving down the group.
Show Answer
Answer
Nitrogen exists as diatomic molecule
Show Answer
Answer
| White phosphorus | |
|---|---|
| It is a soft and waxy solid. It possesses a garlic smell. | It is a hard and crystalline solid, without any smell. |
| It is poisonous. | It is non-poisonous. |
| It is insoluble in water but soluble in carbon disulphide. | It is insoluble in both water and carbon disulphide. |
| It undergoes spontaneous combustion in air. | It is relatively less reactive. |
In both solid and vapour states, it exists as a  |
It exists as a chain of tetrahedral  |
Show Answer
Answer
Catenation is much more common in phosphorous compounds than in nitrogen compounds. This is because of the relative weakness of the
Show Answer
Answer
On heating, orthophosphorus acid
Show Answer
Answer
Show Answer
Answer
The elements of group 16 are collectively called chalcogens.
(i) Elements of group 16 have six valence electrons each. The general electronic configuration of these elements is
(ii) Oxidation state:
Asthese elements have six valence electrons
(iii) Formation of hydrides:
These elements form hydrides of formula
Show Answer
Answer
Oxygen is smaller in size as compared to sulphur. Due to its smaller size, it can effectively form pÃâ,
(Hint: Consider lattice energy factor in the formation of compounds).
Show Answer
Answer
Stability of an ionic compound depends on its lattice energy. More the lattice energy of a compound, more stable it will be.
Lattice energy is directly proportional to the charge carried by an ion. When a metal combines with oxygen, the lattice energy of the oxide involving
Show Answer
Answer
Freons or chlorofluorocarbons (CFCs) are aerosols that accelerate the depletion of ozone. In the presence of ultraviolet radiations, molecules of CFCs break down to form chlorine-free radicals that combine with ozone to form oxygen.
Show Answer
Answer
Sulphuric acid is manufactured by the contact process. It involves the following steps:
Step (i):
Sulphur or sulphide ores are burnt in air to form
Step (ii):
By a reaction with oxygen,
Step (iii):
This oleum is then diluted to obtain
In practice, the plant is operated at 2 bar (pressure) and
Show Answer
Answer
Sulphur dioxide causes harm to the environment in many ways:
1. It combines with water vapour present in the atmosphere to form sulphuric acid. This causes acid rain. Acid rain damages soil, plants, and buildings, especially those made of marble.
2. Even in very low concentrations,
3. It is extremely harmful to plants. Plants exposed to sulphur dioxide for a long time lose colour from their leaves. This condition is known as chlorosis. This happens because the formation of chlorophyll is affected by the presence of sulphur dioxide.
Show Answer
Answer
The general electronic configuration of halogens is
Show Answer
Answer
Fluorine forms only one oxoacid i.e., HOF because of its high electronegativity and small size.
Show Answer
Answer
Both chlorine and oxygen have almost the same electronegativity values, but chlorine rarely forms hydrogen bonding. This is because in comparison to chlorine, oxygen has a smaller size and as a result, a higher electron density per unit volume.
Show Answer
Answer
Uses of
(i) It is used for purifying water.
(ii) It is used as a bleaching agent.
Show Answer
Answer
Almost all halogens are coloured. This is because halogens absorb radiations in the visible region. This results in the excitation of valence electrons to a higher energy region. Since the amount of energy required for excitation differs for each halogen, each halogen displays a different colour.
Show Answer
Answer
(i)
(ii)
Show Answer
Answer
(i)
(ii)
Show Answer
Answer
Neil Bartlett initially carried out a reaction between oxygen and
Later, he realized that the first ionization energy of oxygen
(i)
(ii)
(iii)
(iv)
(v)
Show Answer
Answer
Let the oxidation state of
(i)
(ii)
(iii)
(iv)
(v)
(i)
(ii) Chlorine gas is passed into a solution of
Show Answer
Answer
(i)
(ii)
Show Answer
Answer
Show Answer
Answer
Total electrons
In
CIF acts like a Lewis base as it accepts electrons from
Show Answer
Answer
(i)
(ii)
(i)
(ii)
(iii)
Show Answer
Answer
(i) Bond dissociation energy usually decreases on moving down a group as the atomic size increases. However, the bond dissociation energy of
(ii)
The bond dissociation energy of
(iii)
On moving from nitrogen to bismuth, the size of the atom increases while the electron density on the atom decreases. Thus, the basic strength decreases.
(i)
(ii)
(iii)
(iv)
Show Answer
Answer
(i)
(ii)
(iii)
Show Answer
Answer
(i)

(ii)
XeF

(iii)

Show Answer
Answer
Noble gases do not form molecules. In case of noble gases, the atomic radii corresponds to van der Waal’s radii. On the other hand, the atomic radii of other elements correspond to their covalent radii. By definition, van der Waal’s radii are larger than covalent radii. It is for this reason that noble gases are very large in size as compared to other atoms belonging to the same period.
Show Answer
Answer
Uses of neon gas:
(i) It is mixed with helium to protect electrical equipments from high voltage.
(ii) It is filled in discharge tubes with characteristic colours.
(iii) It is used in beacon lights.
Uses of Argon gas:
(i) Argon along with nitrogen is used in gas-filled electric lamps. This is because Ar is more inert than
(ii) It is usually used to provide an inert temperature in a high metallurgical process.
(iii) It is also used in laboratories to handle air-sensitive substances.






