magnetism-and-matter Question 7
Question: Q. 1. An electron of mass
U] [Delhi II 2017, 2014]
Show Answer
Solution:
Ans. (i) Behaviour of revolving electron as a tiny magnetic dipole.
(ii) Proof of the relation
(iii) Significance of negative sign Electron, in circular motion around the nucleus constitutes a current loop which behaves like a magnetic dipole.
Current associated with the revolving electron :
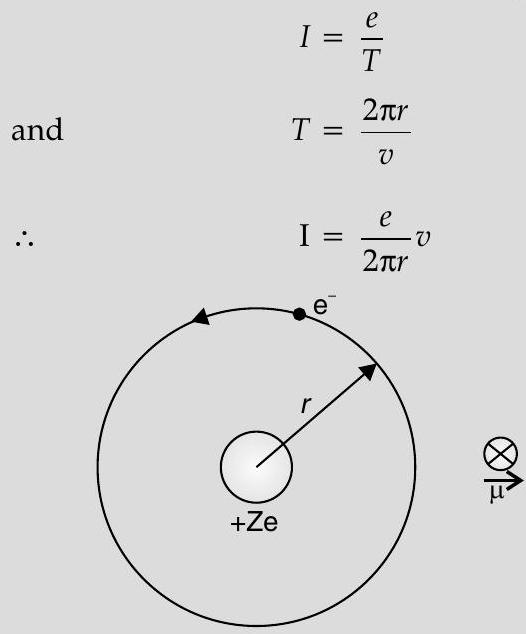
Magnetic moment of the loop,
Orbital angular momentum of the electron,
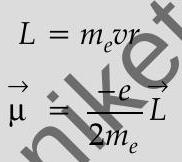
-ve sign signifies that the angular momentum of the revolving electron is opposite in direction to the magnetic moment associated with it.
[CBSE Marking Scheme 2017]






