The Atomic Nucleus Fission and Radioactivity
The Atomic Nucleus Fission and Radioactivity
Solar Energy; T = 1.5×107K
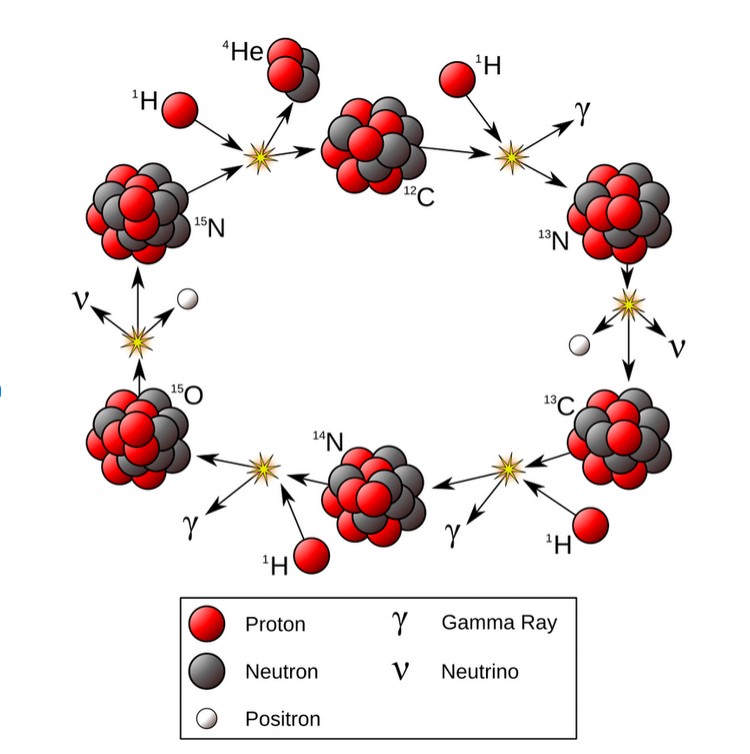
Carbon Cycle
Death of Stars
The Atomic Nucleus Fission and Radioactivity
Mass Versus Life Time
| Mass (solar masses |
Time (years) |
| 60 |
3 million |
| 30 |
11 million |
| 10 |
32 million |
| 3 |
370 million |
| 1.5 |
3 billion |
| 1 |
10 billion |
| 0.1 |
1000s billions |
The Atomic Nucleus Fission and Radioactivity
Mass Versus Life Time
More on stability
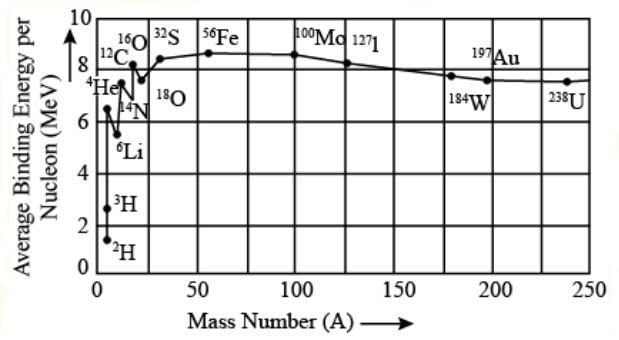
Binding energy per nucleon along the “valley of stability”
| Fusion |
Fission |
| generates |
generates |
| energy |
energy |
More phenomena
- Fission
- Radioactivity
- Gamma decay
The Atomic Nucleus Fission and Radioactivity
Nuclear Fission
Spontaneous fission
Break up into smaller nuclei
Condition: AZ2>47
Example
235U→140Xe+92Sr+3n
Energy liberated: 173 MeV
Catch: Probability for decay ∼10−11 per second!
The Atomic Nucleus Fission and Radioactivity
Nuclear Fission
A⟶X1+X2+He
+n1β+n2γ
r1+r2=n1
The Atomic Nucleus Fission and Radioactivity
Radioactivity
Spontaneous probabilistic process
Beta decay
β−≡e−;β+≡e+
α decay (alpha ≡4He)
γ decay (De-excitation)
The Atomic Nucleus Fission and Radioactivity
Gamma Decay
Electromagnetic
γ decay
De-excitation of Nuclei
Example:
10Be⋆→10Be+γ
56137Ba⋆→56137Ba+γ
No change in A,Z.
The Atomic Nucleus Fission and Radioactivity
Gamma Decay
Atoms Hydrogen
n=3
n=2
10.2 ev
n=1
413.6=3.4
13.6−3.4=10.2
The Atomic Nucleus Fission and Radioactivity
Alpha Decay
Compound Nucleus
α−Particle =24He
M(94240Pu)→M(92236U)+M(24He)
MPUc2>MUC2+MHec2+
[MP−(MD1+MHe)]c2=Q
Q−Factor
The Atomic Nucleus Fission and Radioactivity
Universal Features of Beta Decay
Neutrino Accompaniment
ZAX→Z+1AY+e−+Vˉ
ZAX→Z−1AY+e++V
Beta Decay
α decay: the 4 particles (2n+2p) were inside the nucleus.
(2n+2p)− There is no production as such
The Atomic Nucleus Fission and Radioactivity
Universal Features of Beta Decay
Beta decay
zAX
z→ Protons
A−z→ Neutrons
There are no β− or β+
n⟶p+e−+νˉ
p∗⟶n+e++ν
The Atomic Nucleus Fission and Radioactivity
Examples of Beta Decay
n→p+e−+vˉ
14C→14N+e−+vˉ
10C→10B+v+e+
Fundamental Process
p⟶n+e++ν
${m_p
Hydrogen is stable
p∗→n+e++ν
The Atomic Nucleus Fission and Radioactivity
More Examples
Chain reaction
90232Th→88228Ra+24He
88228Ra→89228Ac+e−
89228Ac→90228Th+e−
90228Th→88224Ra+24He
Uranium Chain
The Atomic Nucleus Fission and Radioactivity
Uranium Chain
Credit: Freidlander et al
Laws of radioactivity
Important Features
Law of Large Numbers
Satistical in Nature
Only Probability matters
Gave rise to Quantum Mechanics!
The Atomic Nucleus Fission and Radioactivity
Law of Exponential Decay
Decay Rate depends on the Population at that Instant
−R≡dtdN(t)
=−λN(t);λ>0
R is also called activity.
The Atomic Nucleus Fission and Radioactivity
Law of Exponential Decay
Units of R : Becquerel (SI);
Curie (Ci)=3.7×1010 Becquerel
dtdN(t)=−λN(t)
R(t)≡λN(t)
R(t)−activity
The Atomic Nucleus Fission and Radioactivity
Law of Exponential Decay
Activity decreases with time
R(t1)=λN(t1)
R(t2)=λN(t2)
t2>t1
R(t1)R(t2)=N(t1)N(t2)<1
The Atomic Nucleus Fission and Radioactivity
Law of Exponential Decay
−dtdN=R(t)=λ N(t)
N(t)→ dimension less.
[λ]=T1
[R]=T1
[R]=T1= S I (s−1)
[R]=T1= Ci
The Atomic Nucleus Fission and Radioactivity
Law of Exponential Decay
λ=SI(s−1): Becquerel
λ=Ci→ Curie
Decay Rate depends on the Population at that Instant
−R≡dtdN(t)=−λN(t);λ>0
R is also called activity.
Units of R : Becquerel (SI);
Curie (Ci)=3.7×1010 Becquerel
The Atomic Nucleus Fission and Radioactivity
Two Concepts
Half Life and Mean life
T1.2=λln2≡λ0.693
τ=λ1
The Atomic Nucleus Fission and Radioactivity
Law of Exponential Decay
Solution of decay equation
dtdN(t)=−λN(t)
∫0tNdN=−λ∫0tdt′
lnN(0)N(t)=−λt⇒N(t)=N0e−λt
N0e−λt= Exponential decay Not a linear decay
The Atomic Nucleus Fission and Radioactivity
Half life (T1/2)
N(t)=N0e−λt
t=0,N=N0
t=T1/2;N=2N0
T1/2= half-life
The Atomic Nucleus Fission and Radioactivity
Law of Exponential Decay
N(T1/2)=N0e−λT1/2=2N0
e−λT1/2=21
⇒T1/2=λln2
[∴λln2= Natural ]
The Atomic Nucleus Fission and Radioactivity
Mean Life
When would all the particles decay if the rate were the same at t=0 ?
Nt=N0−(λN0)t
And N=0 when
t=λ1≡τ
Radioactivity: meanlife
Extrapolation
The Atomic Nucleus Fission and Radioactivity
Decay Chain
Sequential Process
A1(λ1)→A2(λ2)→⋯An
dtdN1=−λ1N1(t)
dtdN2=λ1N1(t)−λ2N2(t)
And so on
The Atomic Nucleus Fission and Radioactivity
Law of Exponential Decay
Multiple Modes
A given source has multiple decay modes
dN(t)=−{λ1+λ2+⋯λn} N(t)dt
The ratios λjλi give the relative decay rates for various daughters

