JEE Main Solved Paper 2017 Question 24
Question: Which of the following molecules is least resonance stabilized? JEE Main Solved Paper-2017
Options:
A) 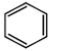
B) 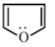
C) 
D) 
Show Answer
Answer:
Correct Answer: B
Solution:
- The molecule in the option (B) is least resonance stabilised. This is because, the three C=C double bonds are not part of benzene ring (i.e, they do not form alternate single and double bond system in a ring system). All the carbon atoms are not sp2 hybridised. Hence, this molecule is not aromatic. All the remaining molecules are aromatic which leads to delocalisation and obeys Huckels rule.










