JEE Main On 8 April 2017 Question 11
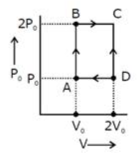
Question: An engine operates by taking n moles of an ideal gas through the cycle ABCDA shown in figure. The thermal efficiency of the engine is - (Take $ C _{v}=1.4R, $ where R is gas constant) [JEE Online 08-04-2017]
Options:
A) 0.24
B) 0.15
C) 0.32
D) 0.08
Show Answer
Answer:
Correct Answer: B
Solution:
- $ w=P _0V _0 $ Heat given $ =Q _{AB}=Q _{BC} $ $ =nC _{V}dT _{AB}+mC _{P}dT _{BC} $ $ =\frac{3}{2}(nRT _{B}-nRT _{A})+\frac{5}{2}(nRT _{C}-nRT _{B}) $ $ =\frac{3}{2}(2P _0V _0-P _0V _0)+\frac{5}{2}(4P _0V _0-2P _0V) $ $ =\frac{13}{2}P _0V _0 $ $ n=\frac{w}{Qgiven}=\frac{2}{13}=0.15 $










